Expired medical items can be risky because of changes in their chemical composition; moreover, different expired medications are at risk of sub potent antibiotics and bacterial growth that can lead to serious illness. That’s why the expiration date of any medicine is a thing that can never be ignored. The expiry date is the last day that the manufacturers guarantee about the safety and potency of the medication.
Drug expiration dates exist on medication labels. After the expiration date, there is no guarantee that the medicine will be effective and safe to use. Due to liability and legal concerns, the manufacturers not make any statement about the stability of the drugs and their original expiration dates. With an increased demand for repacking of the drugs, the US food and drug administration (FDA) have provided guidelines about FDA expiration dates.
How does the FDA Determine Expiration Dates?
Since 1979, the FDA has required pharmaceutical manufacturers to give FDA expiration dates on all the medication products. For most of the drug sold in the US, the expiration dates range from 12 to 50 months from the manufactured date. So, the FDA and compounding pharmacy practices to specify the expiration date by appropriate testing.
For unit-dose repackaged drugs, ideal packaging recommends that FDA expiration dates should not exceed six months from the packaging date. Some medications may expire before the expiration date if it is not properly stored; therefore, the FDA always considers pharmacy requirements in MD to properly regulate the expiration dates.
FDA Drug Expiration Date Format
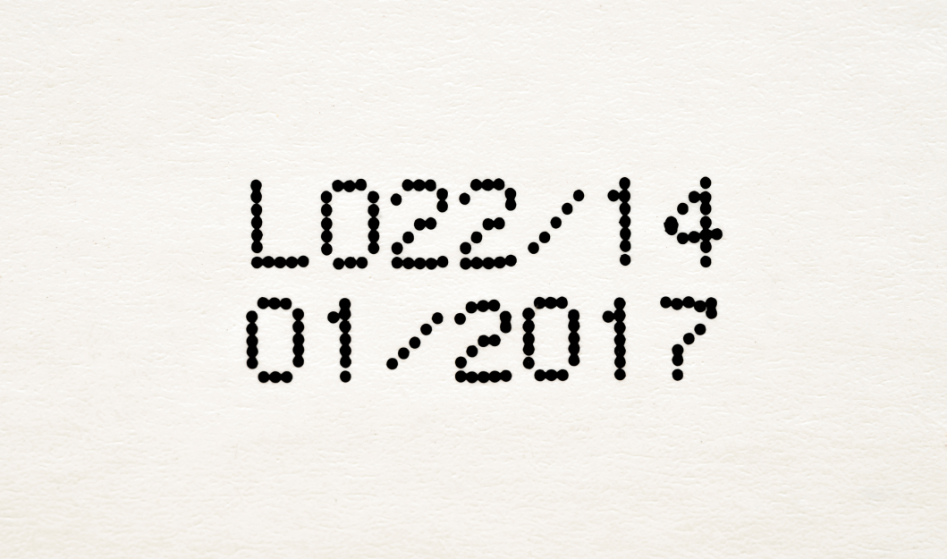
There are some standards regarding the format set by United States Pharmacopeia. The manufacturers can use a variety of abbreviations for dates, months, and years that can be confusing. According to the new FDA drug expiration date format, the expiration date could appear as year/month/day where the year should be in 4 digit format. It will enhance product consistency regulated by USP. These regulations do not apply until September 2023, and you will see a mix of old and new expiration date formats. Therefore, you should need to consider the FDA drug expiration date format.
Drug Expiration Date Regulations and Rules by FDA

The drug expiration date rules are basic guidelines that need consideration by every individual. Below are some FDA drug expiration date regulations to keep the drugs effectively.
Variability
Drugs are in a wide range in terms of stability and dosage. Some drugs like insulin, pediatric liquid antibiotics and some injections expire more quickly as compared to tablets. So, the FDA drug expiration date regulations specify that there is a lot of variability and every product needs to be tested differently.
Stability Testing
The manufacturers generally determine the expiration date and drug’s shelf life through stability testing. This type of testing makes sure the drug’s integrity and potency with the drug’s expiration date; however, still different factors can influence the dates such as storage condition, active ingredients and the container. Every state has different needs, so there are many medicines included in the list of banned medicines in the USA that could not fit into an overall stability among all states.
Storage
One of the FDA drug expiration date rules is the storage condition. The storage should be favorable to keep the medicine last for a long time. Drugs can lose their potency before the expiration date when it is exposed to light, heat, oxygen and humidity; therefore, medicines should be stored in a dark, dry and cool place. In addition to this, you should also keep the medication in its original container.
Closure System
The closure system is one of the basic pharmacy regulations in Maryland. The overall system identifies the packaging according to FDA drug expiration date rules. The container should be ideal enough to enhance the shelf life. Different methods are used to provide an effective closure system to the medicine with proper FDA expiration date extension.
Potential Risks
When you are about to follow FDA drug expiration date rules, it’s important to be aware of the potential risks associated with it. When the drug is degraded, it cannot provide patients the benefits because of its lower strength. In addition, it may yield toxic compounds that can cause customers to experience side effects. You should store drugs properly to keep the patient safe from serious diseases.
Does the FDA Regulate the Expiration Dates?
FDA supports public health programs that involve different partners to extend the expiration dates for drug products. Furthermore, under the Shelf Life Extension Program (SLEP), the FDA conducts testing for products stored in environmentally controlled areas. SLEP is a program that is helpful in the FDA expiration date extension program. The drugs undergo stability testing conducted by the FDA. Furthermore, the appropriate conditions depend upon the drug but it also includes considerations of factors like humidity, temperature and light exposure.
Takeaway

The FDA expiration date is estimated using different testing and manufacturing practices. The current manufacturing regulations of the FDA stipulate that each drug product has an expiration date.
The extended shelf life of the drug depends upon the drug ingredients, storage conditions, light, temperature fluctuations and humidity. Furthermore, the drug items need proper packaging to keep them safe from environmental variations.


